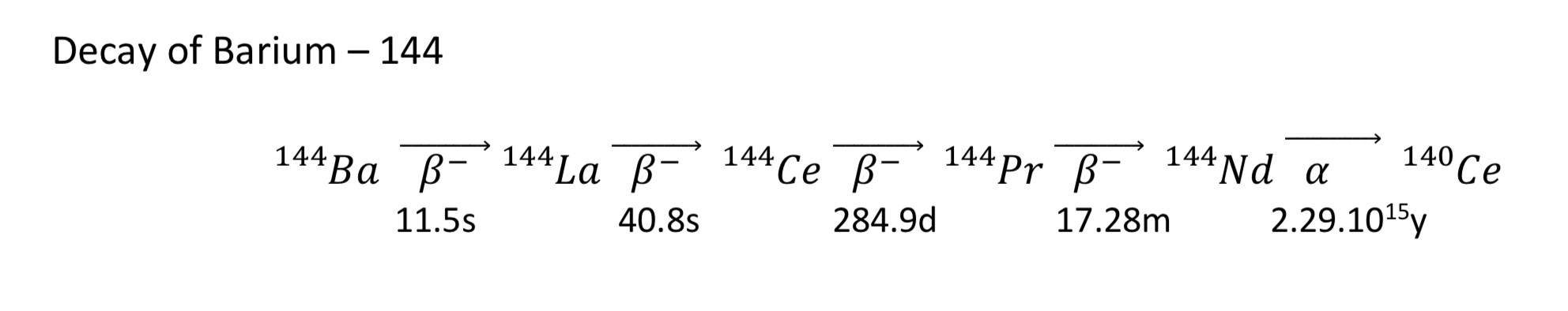In Context: Decay Equations
Always wondered what the point of decay equations were? Read on to learn more about the uses of decay equations, their application in the nuclear industry and write decay equations for a real decay chain that occurs due to nuclear fission.
Each type of decay can be represented using an equation. This shows the decay of the isotope to a new isotope and the emitted radioactive particle.
There are three types of radioactive decay: alpha, beta and gamma. Gamma emission occurs when the nucleus needs to lose excess energy following a decay and does not affect the nucleus (the number of protons and neutrons does not change).
When alpha decay occurs, the nucleus loses 2 protons and 2 neutrons (helium nucleus), and can be written in two different ways:
Beta decay is the changing of a neutron to a proton, with an excess electron emitted from the nucleus. This balances the charge.
Decay equations can be used to model the decay of isotopes released during fission reactions.
A few examples of fission reactions are:
Once fission has occurred, the unstable daughter nuclei produced will decay and then each subsequent nuclei will decay until a stable product has been achieved. Each of the decays can be written as a decay equation. These can be adapted into decay chains.
Decay chains are a more succinct way of writing the series of decays that occur. The respective decays can occur over a few seconds or several hundreds of years. The products cannot be separated, so the longest decay dictates the storage time and the shielding required for radioactive safety of the waste product over time.
For example the decay from Xenon – 144 to Cerium can be written as a decay chain:
Or as a series of decay equations. Here are the last three decays written out:
As an exercise have a go at writing out the 5 decay equations for the decay of Barium - 144 to cerium – 140: